 |
 |
||||||||||
STRUCTURE DETERMINATION BY EXAFS OF Nb-PEROXO-CITRATO COMPLEXES IN AQUEOUS SOLUTION-GEL SYSTEMS
Related publications: M. K. Van Bael, I. Arčon, K. Van Werde, D. Nelis, J. Mullens, L. C. Van Poucke; Structure determination by EXAFS of Nb-peroxo-citrato complexses in aqueous solution-gel systems, Physica Scripta. Vol, T115, (2005), 415-417
Abstract
A structural study of a niobium-peroxo-citrato complex in an aqueous solution, used as a precursor for the synthesis of ceramic oxides by an aqueous solution-gel route is performed by Nb K-edge EXAFS analysis. The results clearly indicate the formation of niobium-peroxo-citrato dimers via Nb-O-Nb links. Nb(V) ions are seven-coordinated including one Nb=O bond of a niobyl group and two Nb-O bonds from a side-on metal-peroxo group. The results are consistent with a previously proposed structural model based on Raman and UV spectroscopy studies.
Introduction
There has been a lot of interest in the preparation of electroceramic metal oxides containing niobium by solution techniques. From an economical and ecological point of view, aqueous routes are preferred. The aqueous solution-gel route involves the formation of a gel network by hydrolysis, condensation and complexation reactions in an aqueous solution of metal salts and chelating or bridging ligands [1-3]. Drying and a suitable heat treatment convert the amorphous gel into the desired ceramic oxide.
Aqueous solution chemistry of high valent metals such as Nb(V) is however very complicated because they are extremely sensitive towards hydrolysis, leading to precipitation and undesired phase segregation. Despite the additional fact that the only available water-soluble Nb compound - an oxalate - is not appropriate for gel formation and combination with other metal ions, we succeeded to transform it into a water-soluble Nb precursor, suitable for gel formation, by reaction with peroxide and complexation with citric acid.
In order to understand the mechanism of the solution-gel processes on the molecular level it is crucial to know the structure of the Nb complex formed in the precursor solution. Up to date only one model was proposed for a niobium-peroxo-citrato complex in aqueous solution by Narendar and Messing [4], based on Raman and UV spectroscopy studies of the solution and additional IR data on the condensed phase. However, to our knowledge, there are no XRD or other direct data on the structure of the Nb complex.
The objective of this work is to study the structure of the Nb-peroxo-citrato complex in the aqueous solution by Nb K-edge EXAFS. We analyse the local niobium environment and compare the structural results with the model proposed by Narendar and Messing.
Experimental
The aqueous niobium solution was synthesized according to the procedure of Nelis et al. [2] by first oxidizing niobium(V) ammonium oxalate with hydrogen peroxide in an aqueous solution of citric acid. The pH is then raised to 7.5 with ammonia which allows the citrate groups to co-ordinate the metal ions and to form a stable water-soluble Nb(V)-peroxo-citrato complex.
The Nb K-edge EXAFS spectrum of the solution was measured at the BM20 beamline of the ESRF synchrotron radiation facility. The station provided a Si(111) fixed-exit double-crystal monochromator with about 3 eV resolution at the Nb K-edge. Harmonics were effectively suppressed (below 8x10![]() ) by a slight detuning of the monochromator crystals, keeping the intensity at 70 % of the rocking curve with the beam stabilization feedback control. The beam was focused using two mirrors with silicon reflecting stripes on a silicon substrate. The first mirror is bent for horizontal collimation of the synchrotron radiation from the bending magnet on the monochromator, while the second mirror, placed after the monochromator, allows a vertical focusing of the monochromatic beam onto the sample. The beam size on the sample was 1mm x 8 mm.
) by a slight detuning of the monochromator crystals, keeping the intensity at 70 % of the rocking curve with the beam stabilization feedback control. The beam was focused using two mirrors with silicon reflecting stripes on a silicon substrate. The first mirror is bent for horizontal collimation of the synchrotron radiation from the bending magnet on the monochromator, while the second mirror, placed after the monochromator, allows a vertical focusing of the monochromatic beam onto the sample. The beam size on the sample was 1mm x 8 mm.
The solution was inserted in a polyethylene cuvette with a path length of 8.5 mm. The Nb concentration of 0.15 M was chosen to obtain an optimal absorption thickness of about 1 above the Nb K-edge. The intensity of the incident and transmitted beam was measured with Ar filled ionisation cells. The standard stepping progression within [-250 eV - 1000 eV] region of the Nb K-edge was adopted for EXAFS spectra with an integration time of 2s/step. The exact energy calibration is established with simultaneous absorption measurements on an Nb metal foil.
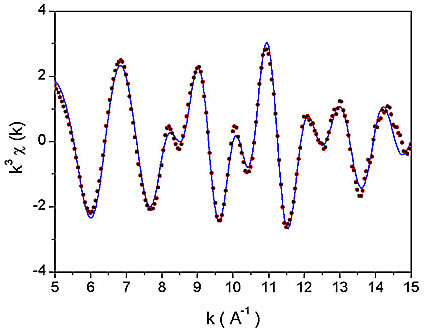
Figure 1: The k![]() weighted Nb K-edge EXAFS spectra of Nb-peroxo-citrato complex in the solution. Dots - experiment; solid line - EXAFS model.
weighted Nb K-edge EXAFS spectra of Nb-peroxo-citrato complex in the solution. Dots - experiment; solid line - EXAFS model.
Nb EXAFS spectrum (Fig. 1) was analyzed by the University of Washington analysis programs using FEFF6 code for ab initio calculation of scattering paths [5,6]. Nb neighbour shells are discerned in the k![]() weighted Fourier transforms of the EXAFS spectrum shown on Fig. 2 together with a best-fit EXAFS model. A very good fit is found in the R range from 1.3
weighted Fourier transforms of the EXAFS spectrum shown on Fig. 2 together with a best-fit EXAFS model. A very good fit is found in the R range from 1.3 ![]() to 4.0
to 4.0 ![]() with seven oxygen atoms in the first coordination shell, and one Nb atom and several carbon atoms in a second shell. The quality of the fit is shown also on Fig. 1. A complete list of best-fit parameters is presented in Table 1.
with seven oxygen atoms in the first coordination shell, and one Nb atom and several carbon atoms in a second shell. The quality of the fit is shown also on Fig. 1. A complete list of best-fit parameters is presented in Table 1.
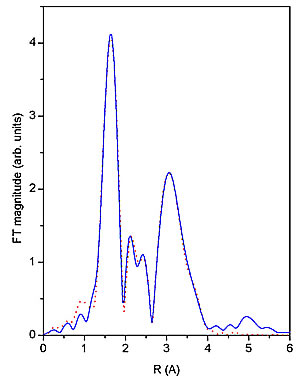
Figure 2: The k![]() weighted Fourier transforms of Nb EXAFS spectra of Nb-peroxo-citrato complex in the solution calculated in the k range of 5
weighted Fourier transforms of Nb EXAFS spectra of Nb-peroxo-citrato complex in the solution calculated in the k range of 5 ![]() to 15
to 15 ![]() . Solid line- experiment, dotted line - EXAFS model.
. Solid line- experiment, dotted line - EXAFS model.
Neigh. |
N |
R (A) |
s2 (A2) |
O |
0.8(1) |
1.75(2) |
0.005 (2) |
O |
2.6(4) |
2.03 (1) |
0.004(2) |
O |
2.9(4) |
2.59(1) |
0.007(3) |
C |
1.5(4) |
2.98(3) |
0.008(3) |
Nb |
1.0(1) |
3.30(1) |
0.004(1) |
C |
5(1) |
3.76(2) |
0.004(2) |
C |
2(1) |
4.08(2) |
0.004(2) |
Table 1: Structural parameters of the nearest coordination shells around Nb atom in aqueous solution of the Nb-peroxo-citrato complex : type of the neighbour atom, number N, distance R, and Debye-Waller factor ![]() . Uncertainties of the last digit are given in the parentheses.
. Uncertainties of the last digit are given in the parentheses.
To describe the first coordination shell we included in the model three different Nb-O distances that can be expected in similar metal-peroxo complexes [7-11]. Indeed, we found one oxygen at a short distance of 1.75 ![]() , corresponding to niobyl group (Nb=O) which is consistent with bond lengths of metal-oxo groups, e.g. Mo=0
, corresponding to niobyl group (Nb=O) which is consistent with bond lengths of metal-oxo groups, e.g. Mo=0
(1.66 ![]() ) [8] and V=O (1.60
) [8] and V=O (1.60 ![]() ) [10]. The remaining six oxygens are found to be distributed into two groups: first three centered at the distance of 2.03
) [10]. The remaining six oxygens are found to be distributed into two groups: first three centered at the distance of 2.03 ![]() and the remaining three at a much longer distance of 2.59
and the remaining three at a much longer distance of 2.59 ![]() . A metal-oxygen distance of about 2.0
. A metal-oxygen distance of about 2.0 ![]() is characteristic for side-on metal-peroxo bonds [12, 13], while the longer distance indicates the presence of carboxylate oxygens coordinated to Nb. Due to large correlations between fitting parameters it was not possible to obtain a more precise distribution of oxygen neighbours in the first shell. Even at this level of details we obtained relatively large uncertainties for number of neighbours and Debye-Waller factors.
is characteristic for side-on metal-peroxo bonds [12, 13], while the longer distance indicates the presence of carboxylate oxygens coordinated to Nb. Due to large correlations between fitting parameters it was not possible to obtain a more precise distribution of oxygen neighbours in the first shell. Even at this level of details we obtained relatively large uncertainties for number of neighbours and Debye-Waller factors.
Similarly, due to high correlations of the second shell parameters, we were not able to determine the exact number of carbon atoms, which were found at non-equal distances in that shell. However, the Nb neighbour parameters could be reliably determined since they were not affected by the correlations.
Discussion
The structural parameters obtained by Nb EXAFS can be compared with the proposed model for the niobium-peroxo-citrato complex in aqueous solution by Narendar and Messing [4]. Their model consists of a dimeric structure in which each Nb(V) ion is surrounded by seven oxygens, one from a niobyl group, two from a side-on peroxo group and four from citrate ligands.
In our case the presence of a single Nb neighbour at 3.30![]() clearly indicates the formation of dimers via Nb-O-Nb links. We also find seven-coordinated Nb with one short Nb=O bond of a niobyl group. Two of the three oxygens at about 2.0
clearly indicates the formation of dimers via Nb-O-Nb links. We also find seven-coordinated Nb with one short Nb=O bond of a niobyl group. Two of the three oxygens at about 2.0![]() may be ascribed to side-on metal-peroxo bonds, for which we have additional evidence from the UV spectrum of the solution, showing the characteristic ligand-metal charge-transfer band at 255-260 nm [14]. The remaining four oxygens most probably originate from carboxylate and a-hydroxy groups of citric acid (C(OH)(COOH)(CH
may be ascribed to side-on metal-peroxo bonds, for which we have additional evidence from the UV spectrum of the solution, showing the characteristic ligand-metal charge-transfer band at 255-260 nm [14]. The remaining four oxygens most probably originate from carboxylate and a-hydroxy groups of citric acid (C(OH)(COOH)(CH![]() COOH)
COOH)![]() ). The hydroxy coordination is expected because the complex synthesized with a modified ligand without the a-OH group is not stable. The oxygens at a relatively long distance of about 2.
). The hydroxy coordination is expected because the complex synthesized with a modified ligand without the a-OH group is not stable. The oxygens at a relatively long distance of about 2. ![]() may be ascribed to carboxylate groups for which a closer approach is hindered by increased strain within the citrate ligands. The carbon atoms in the second shell found at 3.0
may be ascribed to carboxylate groups for which a closer approach is hindered by increased strain within the citrate ligands. The carbon atoms in the second shell found at 3.0![]() , 3.8
, 3.8![]() and 4.1
and 4.1![]() originate from citrate functional groups.
originate from citrate functional groups.
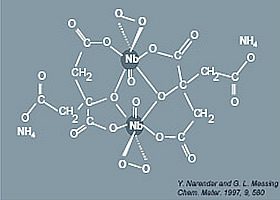
Figure 3: Structural model of Narendar and Messing.
Our results are consistent with the structural model of Narendar and Messing [4]. Other structural studies of metal-peroxo-carboxylato complexes in condensed phases based on IR spectroscopy, thermal analysis and X-ray diffraction [4,7-15] also support our findings: most of them reveal a structure with a pentagonal bipyramid coordination of the metal which is then sp![]() d
d![]() hybridized. Additionally, in many cases dimer units are formed in amorphous precipitates or in a crystalline form of similar peroxo- carboxylato complexes [4,9-11]. However, we find some differences compared to the XRD data obtained on crystalline samples with the same dimeric structural units. In the solution we observed three long Nb-O distances instead of one reported for the crystalline samples of other metal complexes [9-11]. Therefore our results suggest that the dimers may have slightly different structure in solution than in a condensed phase.
hybridized. Additionally, in many cases dimer units are formed in amorphous precipitates or in a crystalline form of similar peroxo- carboxylato complexes [4,9-11]. However, we find some differences compared to the XRD data obtained on crystalline samples with the same dimeric structural units. In the solution we observed three long Nb-O distances instead of one reported for the crystalline samples of other metal complexes [9-11]. Therefore our results suggest that the dimers may have slightly different structure in solution than in a condensed phase.
Acknowledgements
This work was partly financed the by Fund for Scientific Research of Flanders (F.W.O.-Vlaanderen) (Belgium) via the research program G0257.95 CRG/DUBBLE. We acknowledge the European Synchrotron Radiation Facility for provision of synchrotron radiation facilities (experiment ME-246) and we would like to thank T. Reich and A. Bauer for assistance in using the Radiochemistry and materials research ROBL-CRG beamline (BM20).
I. Arčon is supported by the Slovenian Ministry of Education, Science and Sport. M.K. Van Bael and D. Nelis are respectively a post-doctoral fellow and a research assistent of the F.W.O.-Vlaanderen. K. Van Werde is indebted to the 'Instituut voor de aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen' (I.W.T.).
- Nelis, D., Van Werde, K., Mondelaers, D., Vanhoyland, G., Van den Rul, H., Van Bael, M.K., Mullens, J. and Van Poucke, L.C., J. Sol-Gel Sci. Tech. 26, 1125 (2003)
- Nelis, D., Van Bael, M.K., Van den Rul, H., Mullens, J., Van Poucke, L.C., Vanhoyland, G., D'Haen, J., Laureyn W. and Wouters, D.J., Integr. Ferroelectrics 45, 205 (2002)
- Van Werde, K., Vanhoyland, G., Nelis, D., Mondelaers, D., Van Bael, M.K., Mullens, J. and Van Poucke, L.C., J. Mater. Chem. 11, 1192 (2001)
- Narendar, Y. and Messing, G.L., Chem. Mater. 9, 580 (1997)
- Stern, E.A., Newville, M., Ravel, B., Yacoby, Y. and Haskel, D., Physica B 208 & 209, 117 (1995)
- Rehr, J.J., Albers, R.C. and Zabinsky, S.I., Phys. Rev. Lett. 69, 3397 (1992)
- Djordjevic, C., Wilkins, P.L., Sin, E. and Butcher, R.J., Inorg. Chim. Acta 230, 241 (1995)
- Flanagan, J., Griffith, W.P., Skapski, A.C. and Wiggins, R.W., Inorg. Chim. Acta 96, L23 (1985)
- Djordjevic, C., Gundersen, J.L., Jacobs, B.A. and Sin, E., Polyhedron Commun. 8, 541 (1989)
- Djordjevic, C., Lee, M. and Sin, E., Inorg. Chem. 28, 719 (1989)
- Djordjevic, C., Lee, M. and Sin, E., Inorg.Chim.Acta 233, 97 (1995)
- Bayot, D., Tinant, B. and Devillers, M., Catal. Today 78, 439 (2003)
- Bayot, D., Tinant, B., Mathieu, B., Declerq, J.P. and Devillers, M., Eur. J. Inorg. Chem. 737 (2003)
- Van Werde, K., Ph.D. Thesis - Limburgs Universitair Centrum, Belgium (2003)
- Griffith, W.P. and Wickens T.D., J. Chem. Soc. A, 397 (1968)
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
E-mail:iztok.arcon@p-ng.si Last change: 29-Jun-2006 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||