 |
 |
||||||||||
Exafs determination of the composition and structure of transitional metal aluminides multilayer coatings
Related
publications:
1)
I.
Arčon, M. Mozetič, A. Zalar, A. Kodre, J. Jagielski, Nucl.
Instrum. Methods, B 199 (2003) 222-226
2) M. Mozetič,
A. Zalar, J. Jagielski, I. Arčon, P. Panjan Surface
and Interface Analysis, 34 (2002) 365-368
3)
I.
Arčon, M. Mozetič, A. Kodre, J. Jagielski, A. Traverse, J.
Synch. Radiation 8 (2001) 493-495
Introduction
Transitional metal aluminides are of great interest owing to their
industrial application. The Fe-Al solid solution, in particular,
shows good wear
resistance and excellent resistance to oxidation, sulphidation
and corrosion [1]. Fe-Al intermetallic
phases can be used as heat- and corrosion-resistant
coatings for bulk materials [2,3].
Thin coatings of pure FeAl phase can be prepared in different ways
including co-deposition of both materials, implantation of one
ion species into
the layer of the other metal, and consecutive deposition of Fe
and Al thin films [4,5]. The
multilayers are heated to appropriate temperature in order to obtain
a uniform coating. During the deposition at room
temperature
some migration of Fe into Al occurs. The FeAl phase is reported
to start growing at the temperature of about 200°C, but rapid nucleation
and growth
of FeAl is observed at the temperature of about 300°C. Between
400
and 600°C the FeAl phase is transformed into the Fe3Al lattice.
At 700°C
the ![]() -Fe
phase becomes dominant [5].
-Fe
phase becomes dominant [5].
In this paper we present a study of the phase formation in the
Fe/Al multilayers by ion beam mixing at different temperatures.
The composition
of the coatings is determined by AES depth profiling and the atomic
structure of the coating by Extended X-rays Absorption Fine Structure
(EXAFS).
 |
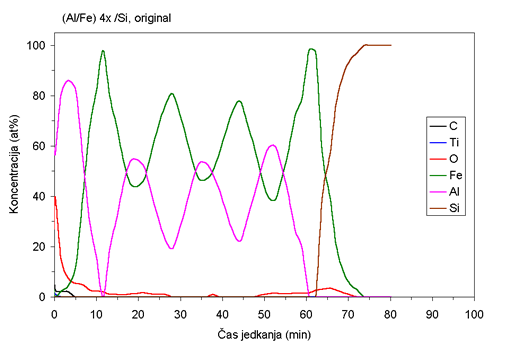 |
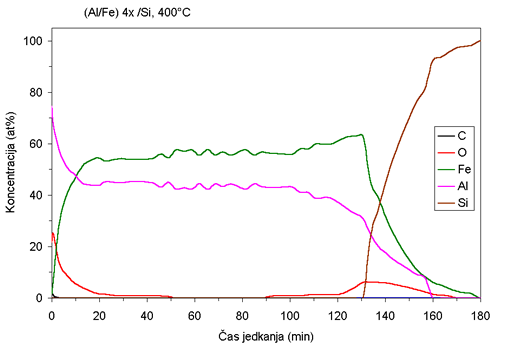
| Fig. 1. AES depth profiles of Fe/Al samples ion beam mixed at room temperature and at 400°C. |
Experiment
Four layers of Fe and Al were alternately sputter-deposited on
well-polished silicon substrates in order to obtain Fe/Al multilayer.
The respective
layer thickness was 20 and 30 nm, to ensure a 1:1 atomic composition
of as-deposited coating. The samples were ion beam mixed with the dose
3x10^15 ions/cm2 of Ar ions with the kinetic energy of 330 keV, at
different sample temperatures between room temperature and 400°C. The
composition
of samples was determined by AES depth profile analysis with the scanning
Auger microprobe (Physical Electronics Ind SAM 545 A).
Fe K-edge EXAFS spectra of the Fe/Al films and a reference Fe foil
were recorded at the X1 station in HASYLAB at DESY (Hamburg, Germany).
A Si(111)
double-crystal monochromator was used with 1 eV resolution at the Fe
k-edge (7112 eV) in combination with fluorescence detection technique
for thin film samples. The EXAFS spectra are obtained as the ratio
of the fluorescence detector signal and the signal of the incident
photon
beam from the ionization cell filled with nitrogen at abient pressure.
Typical measuring time for each fluorescence spectrum was 3 hours.
The EXAFS spectrum of 7 micron thick Fe foil was measured in a standard
transmission
mode.
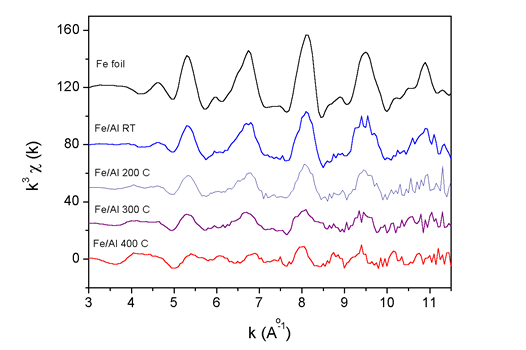 |
| Fig. 2. The k3 weighted Fe K-edge EXAFS spectra measured on the Fe/Al multilayer samples after ion mixing at room temperature (RT) and at 200°C, 300°C and 400°C. For comparison the spectrum of Fe metal foil is added. |
Results and Discussion
AES depth profiles of the as deposited sample and the sample mixed
at 400°C are shown in Fig. 1. Although the deposition was carried
out at
room temperature, the rather wide interfaces between the layers (Fig.
1a) indicated that some migration of atoms between the layers occurred
prior to ion beam mixing. Ion beam mixing at room temperature does
not produce substantial additional migration. However, extensive
migration of atoms between the layers is observed during ion beam
mixing at elevated
temperatures. At 300°C some of the layered structure still persists,
while at 400°C the coating becomes almost uniform (Fig 1b). It is
interesting that the composition of the Fe:Al in the sample mixed
at 400°C is not
1:1 as one would expect from atom ratio in as-deposited samples,
but rather 0.55:0.45. Some aluminum is evidently lost - probably
sputtered
off during the ion beam mixing.
EXAFS analysis is performed with the University of Washington programs
using FEFF6 code for ab initio calculation of scattering paths [6,7].
The basic facts about the structure can be deduced already from the
Fourier transforms of the Fe K-edge spectra (Fig. 2), even before the
detailed
quantitative analysis is performed. We can see that in the thin film
mixed at room temperature Fe exhibits pure bcc crystal structure, same
as in the reference metal foil. However, with increasing substrate
temperature, a gradual change from pure Fe bcc to a new Fe-Al alloy
structure is observed.
The mixing on the atomic scale is therefore more efficient at higher
substrate temperatures as already expected from the AES depth profiles.
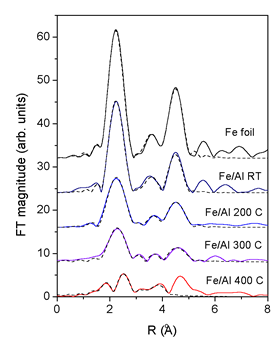 |
| Fig. 3.The k3 weighted Fourier transforms of the Fe K-edge EXAFS spectra (k = 4.5 A-1 to 11.5 A-1) measured on the Fe/Al multilayer samples after ion mixing at room temperature (RT) and at 200°C, 300° C and 400°C. For comparison the spectrum of Fe metal foil is added. Solid line - experiment, dashed line – EXAFS model. |
Quantitative EXAFS analysis is used to determine the ratio of the
two phases in each coating. FEFF model of the bcc crystal
structure of
Fe metal is constructed from the crystallographic data (the lattice
constant
a = 2.87 A [8]). The Fe atom is surrounded by 8 atoms at 2.49
A, 6 at 2.87 A, 12 at 4.06 A, 24 at 4.76 A, and 8 at 4.97 A in
the first
five
consecutive neighbor shells. The FEFF model comprises all single
scattering paths from this shells and all multiple scattering
paths up to 5.7
A. The model is calibrated by the Fe foil spectrum, yielding
an excellent fit for the region from 1.5 to 5.0 A (Fig. 2) with
just five variable
parameters: the lattice expansion ![]() , the amplitude reduction
factor (
, the amplitude reduction
factor (![]() = 0.81(5)), the zero-energy shift DEo, and the
Debye temperature
in modeling the Debye-Waller factors of all paths [6], except
the
first two, for which a separate factor (
= 0.81(5)), the zero-energy shift DEo, and the
Debye temperature
in modeling the Debye-Waller factors of all paths [6], except
the
first two, for which a separate factor (![]() )
is introduced. The shell coordination
numbers and unperturbed radii are fixed at their bcc values.
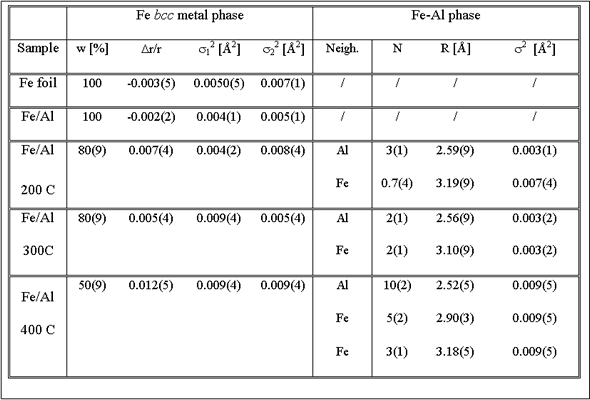
Best fit values of the parameters are included in Table 1.
)
is introduced. The shell coordination
numbers and unperturbed radii are fixed at their bcc values.
Best fit values of the parameters are included in Table 1.
The room temperature Fe/Al EXAFS spectrum is completely described
by the same Fe bcc model as in the Fe metal foil. Self-absorption
effects
of fluorescence measurements, which reduce the EXAFS amplitude
by a factor of 1.7, agree well with the estimate for the given
experimental
setup
by program code ATOMS [9].
EXAFS fit of the spectra of the FeAl coatings treated at higher
temperatures show that an additional aluminum-rich phase is present
beside the metallic
Fe (Table 1, Fig.2). In this phase Fe atoms are coordinated to
Al atoms in the first coordination shell at 2.5 A and Fe atoms
in the second
coordination shell at 3.1 A. Eventual further coordination shells
could not be distinguished
from the signal of the Fe metal phase. In Fe/Al coatings, treated
at 200°C and 300° C the Fe metal phase is still prevailing (80%),
while
at 400 C about half of Fe atoms in the coating are incorporated
in the Fe-Al
phase. In this case EXAFS fit was successful only up to 4 A from
the central Fe atom. The last peak in the spectrum at 4.7 A is
a combination
of single and multiple scattering contributions from both phases
and cannot be reliably modeled. Additionally, in the Fe bcc phase
of the
samples ion beam mixed at higher temperatures, the Debye - Waller
factors are increased, indicating stronger static disorder in
the metallic
Fe bcc phase.
From the structural parameters obtained by EXAFS fit it is not
possible to determine if the Fe-Al phase is fully crystallized
or if only sub-nano
scale crystallites are formed. Likewise, there is not enough information
to draw definite conclusions on the crystal structure of the observed
Fe-Al phase. The obtained local structure around Fe atoms is similar
but not equal to the one in the cubic FeAl crystal [11]. The presence
of cubic Fe3Al phase [11] can be completely excluded.
Table 1. Parameters of the nearest neighbors up to 3 A from the central Fe atom in the Fe foil and in the Fe/Al multilayer samples after ion mixing at room temperature (RT) and at 200°C, 300°C and 400°C.: w – fraction of Fe bcc crystal phase in the sample; (Dr/r) - Fe bcc lattice expansion, R – neighbor distances in Fe-Al phase, and si2 - Debye-Waller factor for individual shells. Uncertainty of the last digit is given in parentheses. |
 |
Conclusions
A study of the phase formation in the Fe/Al multilayers by ion
beam mixing at different temperatures was performed. AES depth
profiles
of the samples
treated at room temperature and at 100, 200, 300 and 400C showed
gradual mixing of the layers with increasing temperature. At
400°C the mixing
was complete. Fe K-edge EXAFS data showed that at room temperature
there was no mixing on the atomic level. At higher temperatures a
new Fe-Al
phase appeared. Up to 300°C the fraction of this phase was only
about 20%, while at 400°C it increased to 50%. Although the AES depth
profile
of the sample mixed at 400°C showed a uniform coating, the mixing
on the atomic level was not complete.
Acknowledgment
Support by the Slovenian Ministry of Education, Science and Sport and
by Internationales Buero BMBF (Germany) is acknowledged. N. Haack
of HASYLAB (Germany) provided expert advice on the X1 beamline operation.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
E-mail:iztok.arcon@p-ng.si Last change: 02-Jun-2006 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||